KEEPING PACE: WHO’S DRIVING DISCOVERY AND WHERE ARE WE HEADED
We’re on overload. It’s not just the deluge of emails, articles, texts and Tweets we have to keep up with to stay current. It’s the complexity of the information and the pace at which it’s rendered obsolete. How do we balance expediency with the need for accuracy and thoughtfulness? How do we prioritize translational research against the need to first understand? Will we crumble under constrained resources, exploding technology and heightened consumer demand, or seize these challenges as opportunities to propel us forward?
As press releases go, this one was pretty standard: Recursion Pharmaceuticals Announces Funding for Rett Syndrome Research. What wasn’t standard was the short email that the company’s founder, Christopher Gibson, Ph.D., received back: “Our daughter is 38 months old—we hope you are successful.” Attached was a photo of a little girl on a tricycle.
For all the miraculous breakthroughs of medicine, the sober reality is that it’s never enough. There will always be diagnoses, treatments and cures that lie just out of reach. But if in the past, desperate families hung their hope on the doctor’s knowledge . . . and waited, today it’s a different story. They’re canvassing the Internet, sharing information on listservs, funding research, advocating for legislative reform and showing up to doctor’s appointments having earned a Google Ph.D. “I can tell you with certainty that nobody cares more about driving research and clinical trials than patients and their families,” says Emily Coonrod, Ph.D., a molecular biologist and personalized health program manager. With their passion, persistence and the power of the Internet, patients are resetting the scientific agenda. “That email changes how I think every day,” says Gibson. Within minutes of receiving it, he forwarded it to his entire team.
“My hope is that we take the best of the past and marry it with new opportunities to take science in directions we never even imagined.”
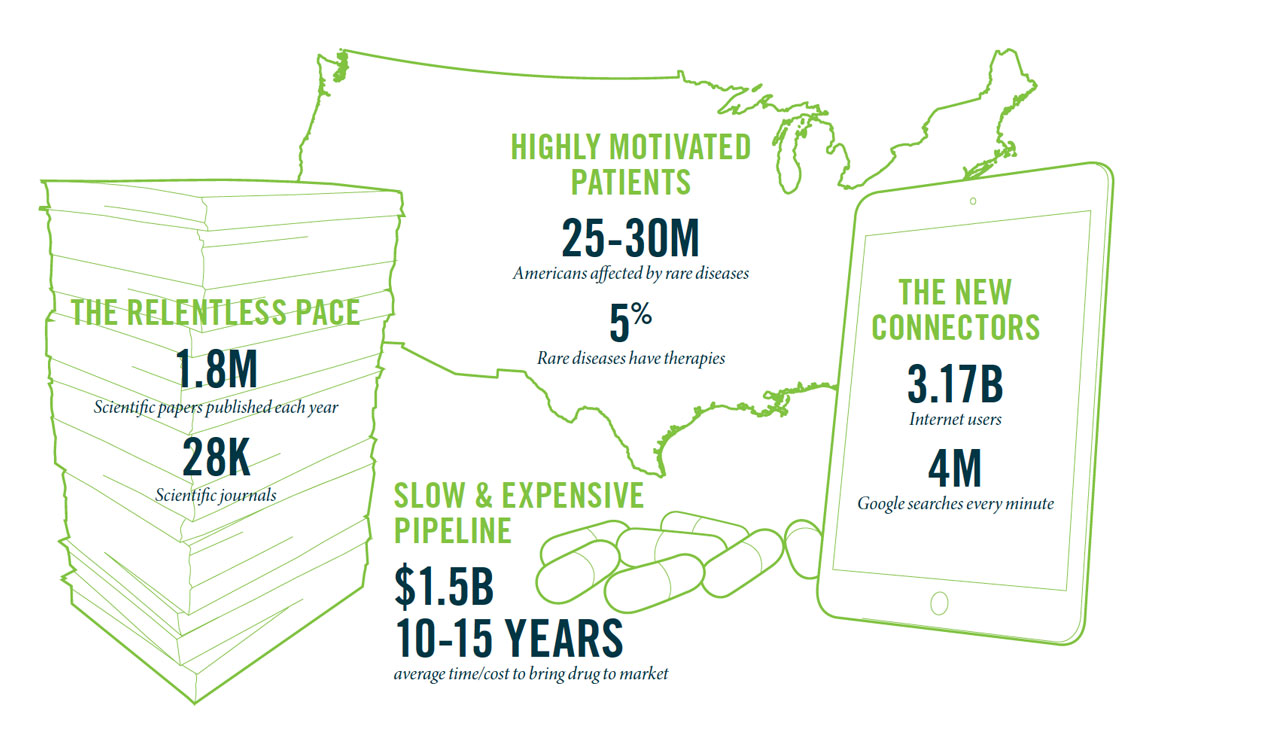
Willard Dere, M.D., Executive Director, Program in Personalized Health“Successful,” as the little girl’s parents hope for Gibson, is a loaded word for today’s scientists and clinicians. With the plummeting price of genome and exome sequencing, and the productivity-enhancing power of big data, it seems we’re closer than ever before to finding solutions to some of our most vexing medical problems—cancer, diabetes, mental illness as well as rare and ill-defined diseases. Yet the advancing flood of scientific knowledge and data is nothing short of mind-boggling. A paper per minute is added to the PubMed database, an index of biomedical abstracts that contains more than 24 million citations. Surgeon and author Atul Gawande, M.D., recently described it this way: The human body has 13 organ systems and 60,000 documented ways they can fail. The job of the scientist and clinician is to figure out how to treat each individual as an “n” of one, with health profiles as unique to them as their fingerprints.
“I feel like I let people down on a regular basis because we can’t get answers for them,” says pediatric geneticist John Carey, M.D., M.P.H., editor-in-chief of the American Journal of Medical Genetics. He estimates that half the patients he sees should be sequenced, but admits that even if insurance agreed to pay for that, it wouldn’t always yield an answer. “I feel disappointment and guilt that I’m not coming through for them.”
Making good on the promise
Call it personalized health or precision medicine, the paradox is the same. Science is infinite and resources are limited. “There are always opportunity costs when you pick one thing over another. Who is responsible for weighing those costs?” says Carrie Byington, M.D., associate vice president for faculty and academic affairs. “How are we going to navigate these competing desires?”
And who will pay for it? “We want to sequence everyone, unleash scientists to explore every avenue and find treatments that are precisely targeted for each unique individual. At the same time, we feel incredibly pressured to keep costs down,” says Senior Vice President Vivian Lee, M.D., P.h.D., M.B.A. “How can we unite these goals?”
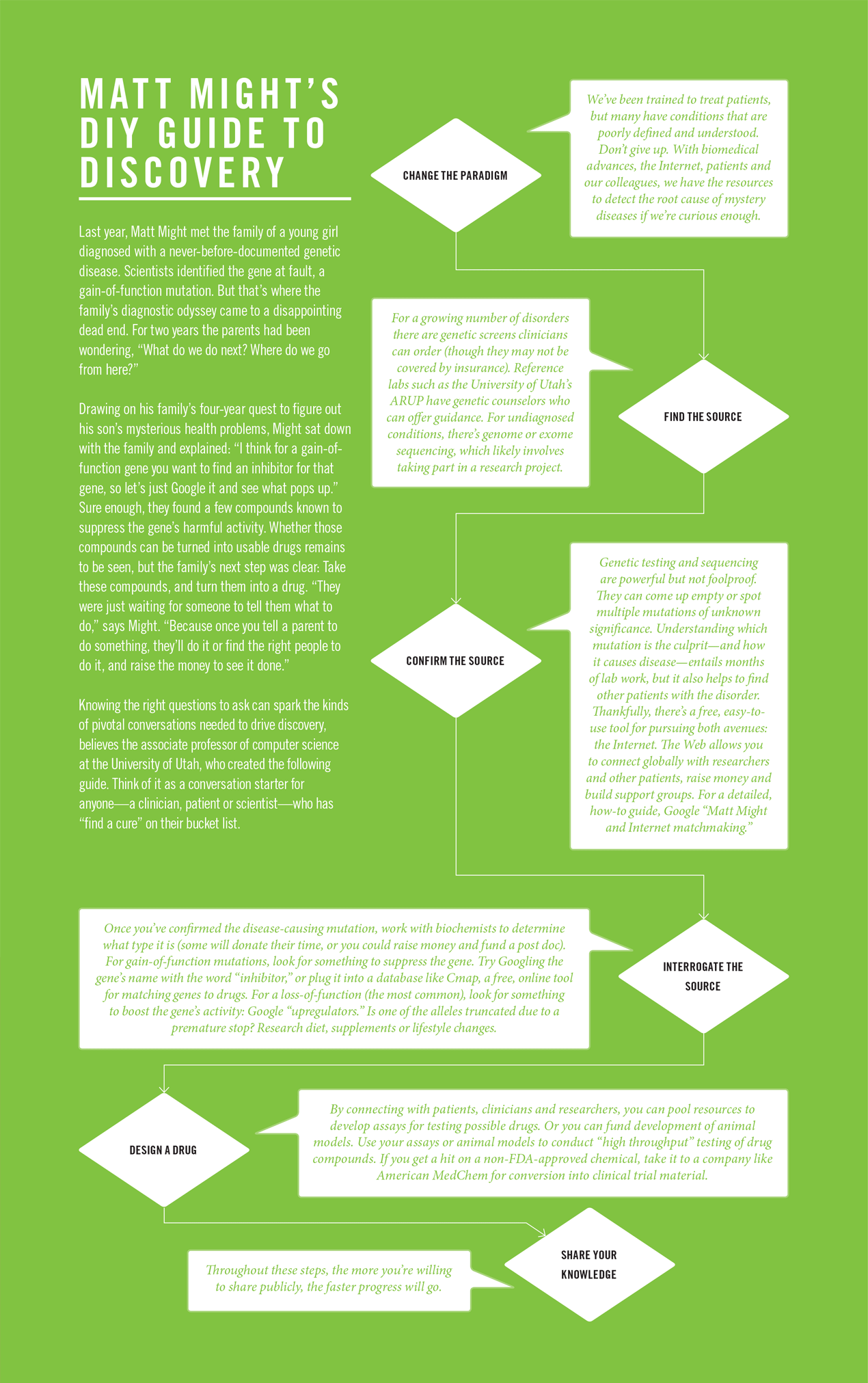
Truth is, no one can predict a priori where the money is best spent. “Science is always a calculated risk,” says citizen scientist Matt Might, Ph.D., whose son’s diagnostic odyssey sparked discovery of a new gene (see page 50). “You can try to invest as strategically as possible, but sometimes your hit, if you’re lucky enough to get one, comes from left field.”
As CEO and Director of Huntsman Cancer Institute, Mary Beckerle, Ph.D., has carefully stewarded countless donations, including the foundational and ongoing gifts of Utah philanthropists Jon and Karen Huntsman, whose ambitious founding goal was to understand and eliminate cancer at its roots. “When there’s a bi-directional understanding and dialogue between the research community and donors, as has been our experience with the Huntsman family, those partnerships can flourish,” says Beckerle, a prolific researcher who has guided the institute for the past decade. “Strong communication insures that donors feel a part of the discovery process, understanding both the exciting potential and the limitations that we, as scientists, face each day.”
Economies of Connection
If the industrial world was all about economies of scale, the post-industrial era is about economies of connection, where progress is driven by how effectively we connect our resources, knowledge and ideas, and how open we are to sharing. “We’re moving away from this feeling that I have to own it and I have to build it myself,” says Byington, who as head of the Center for Clinical and Translational Science makes core resources available to the whole scientific community. Beyond sharing, how do we match up all of this knowledge? “We’ve probably cured every disease five times, we just don’t know it,” says Gibson. Might refers to it as the “unknown known.”
Keeping pace in this fast-paced information age requires us to cultivate a new set of skills. Publish or perish may still be the path to advancing in academia, only now that depends on tapping into a proliferating number of nontraditional sources. One of those is motivated patients. While sometimes their enthusiasm and single focus may seem more distracting than helpful, the truth is they are finding new ways to connect the dots for time-starved clinicians and stressed-out researchers.
“My hope is that we take the best of the past and marry it with new opportunities to take science in directions we never even imagined,” says Willard Dere, M.D., who left the pharmaceutical industry to lead our Program in Personalized Health. We want to get to the future first, says Dean Li, M.D., Ph.D., associate vice president for research. “And to do that, you have to do something no one has done in a way that no one else has done it . . . But no one says you have to do it alone.”
Case Study No. 1: The Patient
Escaping 'Undiagnosed Island'

Not actionable. Matt and Cristina Might would like to see those words stricken from medicine’s vernacular.
To parents of children with ill-defined diseases, those words are disempowering, signaling another dead end in the search for a diagnosis and treatment. They’re also misleading, says Matt Might, Ph.D., associate professor of computer science and adviser to President Obama’s precision medicine initiative. Because in the absence of actionable knowledge, treatments or cures, “science becomes medicine,” he says.
For millions in the rare disease community, the Mights’ diagnostic odyssey has a familiar beginning. The family had just relocated to Salt Lake when concerns deepened about their 6-month-old son, Bertrand. The couple feared autism, the first of a cascade of disorders—each scarier than the last—to be ruled out over four years of EKGs, CT scans, biopsies and blood draws. “You don’t know why your child is hurting or how to stop it,” recalls Matt. In their case, they didn’t know why Bertrand was having seizures and couldn’t cry tears.
They cried plenty for him, says Cristina Might, M.B.A., who put aside her career plans to care for Bertrand and “stitch together” a team of experts. “I spent my time flying him to Duke, Stanford, Baylor; my full time job was just getting answers.” She scoured the Internet for research and clinical trials, sharing everything she learned on a blog: “It’s difficult feeling so alone, so unGoogleable.”
From physical therapy to a stem cell transplant, they explored all avenues. In 2009, they closed Bertrand’s college account to cover medical expenses. Then in 2012, a breakthrough: Duke University scientists who knew the Mights through their transplant attempt invited them to take part in a study of the use of exome sequencing for diagnosing rare diseases. The study unearthed a mutation in the NGLY1 gene that they suspected was the source of Bertrand’s disorder. Matt describes it as having been “rescued from undiagnosed island.”
'Reverse Dragnet'
There was comfort in having something to target with experimental therapies. But they needed to confirm this mutation was the culprit, which traditionally entails months of lab work. Instead, the Mights found success with a user-friendly tool—the Internet. Matt leveraged the social media audience he had amassed through his academic writings and in May, 2012 published a blog post about the “hunt” for his “son’s killer.” He hoped it would act as a “reverse dragnet” to find other patients just like Bertrand.
It worked. The post was seen by millions. Within months the Mights heard from a handful of NGLY1 patients from around the globe, and from researchers who volunteered to test Bertrand’s cells and explore treatments. “Within three months of getting a diagnosis we were trying a compound,” says Matt.
Another series of Google connections coupled with Matt’s relentless self-education pointed to a promising supplement. He found it on Amazon and after taking it himself with no adverse effects—“In my house, I am the FDA,” he says—gave it to Bertrand. Within three days, his son cried his first tear. “It was one small tear but an ocean of science for the disorder because it gave us a clue that this is in some way fundamentally impacting the disorder,” Matt says. Months after taking the supplement, which costs 25 cents a day, Bertrand’s seizures stopped. And his medical expenses have dropped from hundreds of thousands of dollars to less than $30,000 a year.
Their newfound cyber support group replaced “the darkness of isolation” with the “comfort and power of community,” Matt says. Also, sharing stories and clinical data sparked threads for scientists to follow, such as an uncanny ability of NGLY1 patients to ward off viruses, which could be instructive for fighting infectious disease. The families started two nonprofits and supported development of an assay and animal models for testing the 30-plus compounds they’ve identified as possible treatments.
From Citizen Scientist to the White House
The Mights are acutely aware that not everyone has their depth of resources, knowledge or connections. “We had means to travel the world, but most families don’t,” says Cristina. “We’re trying to figure out how we can level the playing field because it’s not fair.”
Matt believes their success is replicable and scalable, which is why he accepts many of the near-daily invitations to share what he’s learned with audiences around the world. Patients can drive science and shape it, he says, offering as proof his own trajectory from concerned father to advising the president’s precision medicine initiative, testifying before Congress and collaborating with University of Utah researchers to develop an NGLY1 therapy. The optimism that drives the Mights doesn’t cloud their view of reality. “I’m very proud of the fact that Matt’s trying to make it better not just for our son,” says Cristina. “Bertrand’s not going to benefit from a lot of the work we’re doing now but other families will and already have. That’s the beautiful thing.”
With Bertrand and two younger children, Cristina’s geographical radius is smaller but her influence is no less great. She is a connector of families, doctors and researchers and a mentor. “It helps to know how the game is played,” she says. “There are little ‘cheat codes’ that make a big difference in finding the right specialist or getting seen sooner.” She’s also co-founded an advocacy group, Utah Rare, and is helping to raise money for an “undiagnosed” clinic at the University of Utah, designed to shepherd families through complicated diagnoses (see Case Study No. 2).
There remain barriers to building a scientific community that’s truly inclusive of patients—from Institutional Review Board (IRB) restrictions on sharing data to the pay walls barring public access to many scientific journals. But it’s getting better, says Matt. “Institutions that figure out how to harness patient energy will be the ones that leapfrog to the front. Patients will seek those places out.”
Case Study No. 2: The Children
A Less-than-Epic Diagnosis

Lately, Lorenzo Botto, M.D., has been intrigued by The Odyssey and its many parallels to the maddening diagnostic journey faced by so many of his patients.
The genetics and pediatrics professor specializes in inborn diseases of metabolism, a cluster of rare, complex disorders that aren’t well understood and often go undiagnosed and thus untreated. By the time most families are referred to him, they’ve spent years bouncing from specialist to specialist, waiting months between appointments and test results only to learn they’ve reached another dead end or been swayed off course. Like Homer’s protagonist, Odysseus, who loses his crew in the first year of his 10-year quest, they battle isolation and despair. “I often hear parents saying, ‘I just wish I knew what my child has, even if it’s a bad thing, so I would know who to connect with,’” says Botto.
The criticism often launched at rare and undiagnosed disease efforts is that we’re throwing too many health care resources at conditions that impact very few children. What critics often don’t realize is the toll on families and the cumulative cost of caring for children who don’t have a diagnosis. “That’s where the frustration and the costs are,” says Botto.
“Families, doctors, nurses, care coordinators—we all agree that the system is not working well for this subset of patients. There are too many steps. It takes too long and it’s far too expensive.”
Lorenzo Botto, M.D., Professor, Department of PediatricsThere are a handful of centers in the U.S. that specialize in rare diseases, but most are research focused. Botto is set on streamlining the clinical side of the equation. “Families, doctors, nurses, care coordinators—we all agree that the system is not working well for this subset of patients,” says Botto. “There are too many steps. It takes too long and it’s far too expensive.” He believes that game-changing tools like whole genome and exome sequencing have the potential to cut costs rather than increase them if deployed at the right time in the diagnostic odyssey. “I believe we can care for these individuals in a way that’s in alignment with the broader goals of the health system.”
The program, still in a pilot phase, will accept children based on their symptoms and the urgency of their health problems. All of their clinical and genetic data will then be gathered into a single, portable and easy-to-understand format that they can take with them. Next, experts will gather in a room to analyze the data, discuss the possible diagnosis and map out a diagnostic plan of attack. They will obtain the opinion of other specialists as needed. “This is a team effort,” says Botto. “We will be combining the collective talents of our master clinicians to push the diagnostic envelope with new tools and processes.” A care coordinator will be a single point of contact for each patient and manage the flow. When no diagnosis is found, the clinic—with its close ties to the University’s Eccles Institute of Human Genetics and the Utah Genome Project—will identify additional research studies in which families can participate.
And who will pay for all of this? Like so many other ideas designed to improve care delivery, Botto admits that much of the care will not be reimbursable by insurers. Instead, an upfront investment by the Department of Pediatrics is funding this pilot program. Long term, Botto is hopeful. By assessing patient and parent satisfaction and tracking time and costs, he hopes to prove to payers that it’s a better, more efficient way to deliver care.
Botto believes that the program will serve as a model for managing more common disorders too. After all, in this age of precision medicine, isn’t every patient arguably an “n of one?” If the goal is to deliver the right treatment to the right person at the right time and for the right cost, this model is scalable, says Botto.
In the meantime “we are joining these families on a journey,” says Edward Clark, M.D., chair of pediatrics. “Maybe we’ll only reach a diagnosis in 30, 40, 50 percent of the cases, but that’s not the end of our commitment to them. We will be their partners throughout their lifetime. Because, if you abandon your patient, they abandon hope.”
Case Study No. 3: The Researcher
Adding Meaning to Minutia

After listening to a colleague give a presentation, Gabrielle Kardon, Ph.D., associate professor of human genetics, couldn’t help herself. Kardon approached the scientist and pointed out that if she took her research in a slightly different direction, she could potentially help kids with congenital diaphragmatic hernia (CDH). “I didn’t used to think about science in terms of clinical outcomes,” says Kardon. “Now it’s almost all I think about.”
As a career biologist, Kardon is a master of minutia, coaxing an understanding of complex physiological processes from their constituent parts: DNA, molecules and cells. The tiny beacons showed her that the diaphragm is greatly weakened when muscle doesn’t form properly, providing new insights into the mechanistic basis of CDH. Nature Genetics and The New York Times featured her work, but Kardon had no idea how important the discovery was until she stepped out of the lab and into the patient community.
A desire to understand what her experiments couldn’t tell her—what it’s like to live, or die, with CDH—led her to Josh Hensley, Utah representative for the support group CHERUBS. He lost two girls to the birth defect, a hole in the diaphragm through which the liver and guts creep into the chest cavity, interfering with the lungs. “I’ll help however I can in the hopes that one day, no one will have to bury their children,” he says. He connected Kardon to the larger CDH community, and through them she learned that even after surgical repair, many deal with a host of complications, not the least of which is the fear that the diaphragm could rupture again at any time.
“Meeting these patients and families has completely changed my perspective on why I do science,” says Kardon, who has since refocused much of her lab toward CDH. That’s why she brought members of her lab to a CDH Awareness Night event at a local baseball stadium. The lab carried balloons proclaiming “No CDH,” and handed them to survivors and their families who carried them with pride. “The students were so excited to meet the kids. And the patients’ parents were amazed that a researcher would go out of their way to spend time with them,” she says.
Now, when making scientific decisions, images of those kids chasing each other around the stadium concourse race through Kardon’s head. Why study the effects of a genetic mutation that, although scientifically interesting, is irrelevant to CDH when she can study ones that ruin lives? “I feel like there is too much riding on the patients,” she says. “It wouldn’t feel like the right decision to ignore projects that could potentially help them.” One of her main objectives is to transform her discoveries into novel interventions.
The community has helped her in unexpected ways as well. In spending time with them, she has become privy to tidbits of their lives that can’t be found in the medical literature. When one parent told her that her son with CDH also had a cleft palate, she thought it was interesting. But after she heard the same tale from five different parents, she knew it was significant. “They’ll mention something in passing, and I’ll say, ‘Wait, what was that again?’” says Kardon. “They don’t even realize they’re a gold mine of valuable information.” Their observations have given her additional clues to the underlying causes of the birth defect.
Her devotion has extended beyond the science, and she has strengthened their voice by adding her unique one, traveling with the community to Washington, D.C. to advocate for additional funding. CDH research receives 1/20th the amount of government support as cystic fibrosis, a birth defect that occurs at about the same rate—one in 2,500. “She is a ray of hope for a community that is desperately seeking answers,” says Hensley.
Case Study No. 4: The Drug Developer
100 Drugs in 10 Years

Christopher Gibson was halfway through medical school and finishing his doctorate in bioengineering when he faced a career-making decision. He and a team of University of Utah scientists had found a shortcut to drug discovery that might quickly identify therapies for dozens of so-called “orphan diseases.”
The 33-year-old could either take the more assured path of finishing medical school to become a cardiothoracic surgeon. Or, he could go the riskier route of starting a drug company, which might fail but had the potential of prolonging the lives of tens of thousands, many of them children. He chose the latter. “There will probably be plenty of talented heart surgeons to fill society’s need, but there was no one on this side doing drug repurposing the way we’re doing it,” explains the newly minted Ph.D.
Develop 100 drugs in 10 years. That’s the ambitious goal set by the founders of Recursion Pharmaceuticals, which harnesses the power of computers and “big data” to quickly and affordably test existing drugs for use with rare diseases. “We’re trying to teach old drugs new tricks,” says Gibson. The conventional path to drug discovery is to identify the biological mechanism behind a given disease and then target it with a drug, testing it first in the lab, then in animals and finally on humans. The entire process can take 10 to 15 years on average and $1 billion or more. Often, despite the best science, the drug fails to work as promised. “For every drug that’s approved by the FDA, there’s usually eight or nine that made it pretty close,” Gibson says. “It’s not a viable business model in the long-term. Drug companies can’t afford it and neither can we as a society.”
Recursion’s strategy is two-fold. Instead of targeting diseases one by one, its assembly-line strategy is to tackle many at once. “We’re trying to take a systems-level approach where we bypass understanding the ins-and-outs of each disease and instead try to recognize what fixes it,” says Gibson. The company manufactures genetic diseases in human cells and then bathes them in drug compounds to see if they can be restored to their normal “healthy” state. And they partner with manufacturers to salvage and repurpose drugs that are collecting dust on shelves. These are often drugs that passed early safety trials but never made it to market, perhaps because they weren’t a perfect match for a particular disease.
At a time when health care is under pressure to contain costs, and federal funding for science is less certain, academic medical centers need to find faster paths to discovery, says Dean Li, M.D., Ph.D., associate vice president for research, in whose lab the start-up was born. “If Chris succeeds at repurposing shelved drug assets, he’ll not only benefit patients. He’ll have rescued dozens or maybe hundreds of drugs from the dustbin of history, along with the tens of thousands of hours and millions of dollars invested in producing them.”
The idea of a pharmaceutical company tackling hundreds of diseases at once is unthinkable for even the largest drug makers. But Recursion has already applied for expedited FDA approval to move forward on a clinical trial of a possible therapy for cerebral cavernous malformation (CCM), a rare hereditary vascular disease that leads to hemorrhagic strokes. The company has also secured nearly $4 million in federal grants and private investments to fund the screening of thousands of drugs across hundreds of genetic diseases.
One of the biggest challenges, says Gibson, is finding ways to bridge the tech part of the equation with the biology, which tends to move more slowly. “We’re constantly balancing urgency with the need for accuracy,” he says. “With technology you can iterate so quickly. But we can’t speed up biology. Cells take time to grow and drugs take time to dose.” There’s no shortage of motivation to be found as a scrappy start-up. For his part, Gibson is driven by the families and patients who are counting on their success. “It frankly just comes down to really caring about what you do.”